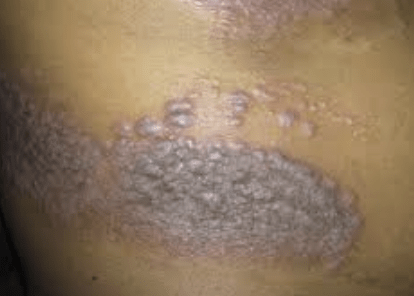
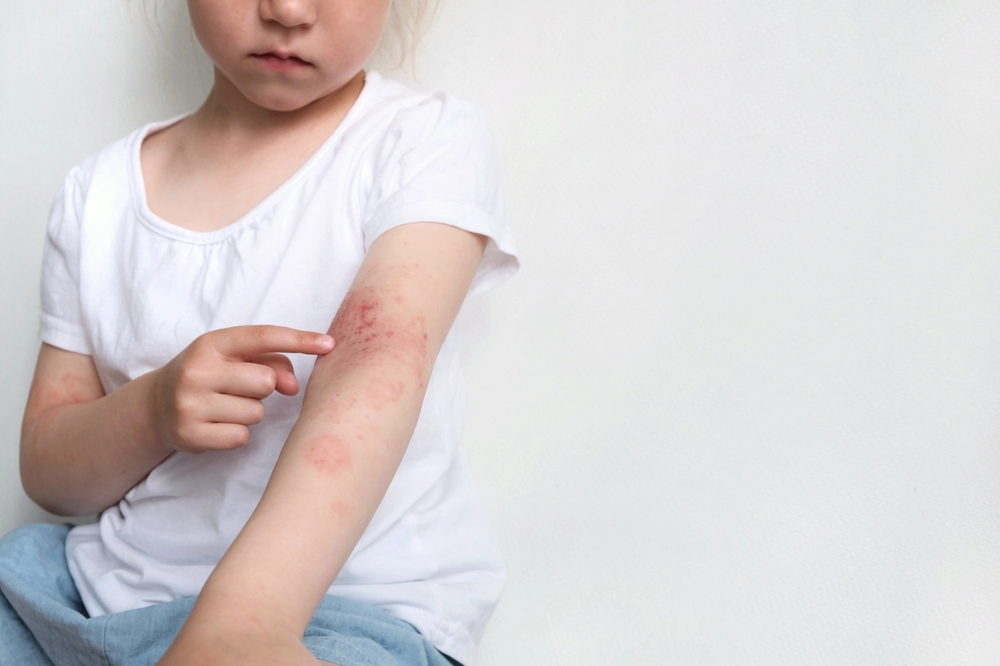
Children with atopic dermatitis (AD)—as well as their families—may experience negative impacts on their quality of life. The phase 3 ADORING 2 trial examined the efficacy and safety of topical tapinarof (VTAMA) cream, 1%, compared with a vehicle cream in patients with AD—including adult patients and pediatric patients as young as 2 years old.
Approximately half of the subjects who were given VTAMA cream achieved clear or almost clear response, also achieving a 2-grade from baseline improvement in Validated Investigator Global Assessment for Atopic Dermatitis at Week 8. Topical tapinarof cream use resulted in high statistical significance in all secondary endpoints, which included considerable improvement in Eczema Area and Severity Index for 59.1% vs 21.2% of patients who received the vehicle. Measured by the Peak Pruritus Numeric Rating Scale, those who used tapinarof cream had significant improvement in itch. Mild or moderate adverse events were reported by the patients. The researchers concluded that tapinarof cream was safe and well-tolerated across all age groups, including patients as young as 2 years old, with no reported safety or tolerability concerns.
Reference: Smith T. Positive phase 3 trial results for tapinarof cream on atopic dermatitis. HCP Live. Updated March 15, 2023. Accessed April 11, 2023. https://www.hcplive.com/view/positive-phase-3-trial-results-tapinarof-cream-atopic-dermatitis
Link: https://www.hcplive.com/view/positive-phase-3-trial-results-tapinarof-cream-atopic-dermatitis