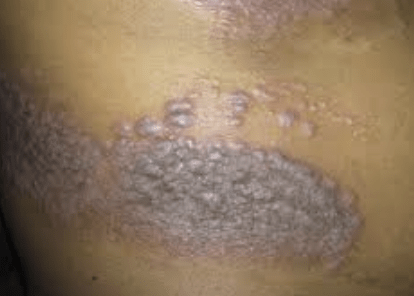
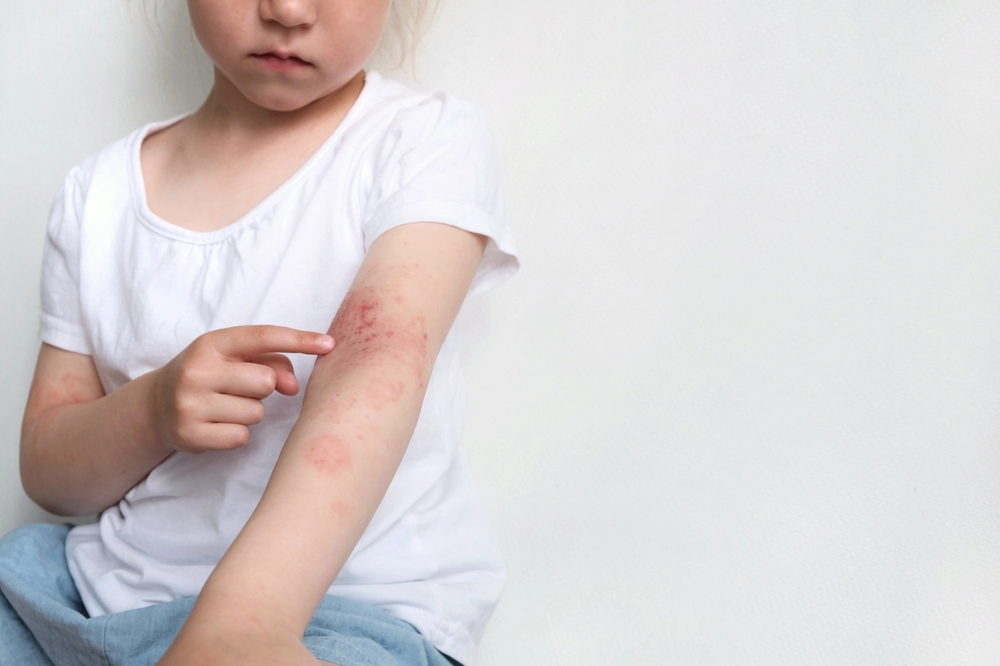
Results from the DERMIS-1 and DERMIS-2 trials were presented at the 2023 American Academy of Dermatology Annual Meeting in New Orleans, Louisiana. The clinical trial program consisted of 2 identical, phase 3, randomized, double-blind, vehicle-controlled, 8-week studies of once-daily roflumilast cream 0.3% in those (≥2 years of age) who had psoriasis occupying 2% to 20% of body surface area (BSA). At 30 testing centers in the United States and Canada, a 52-week phase 2 safety trial was done. The primary endpoint of both DERMIS-1 and DERMIS-2 was an Investigator Global Assessment (IGA) score of almost clear with no less than a 2-grade improvement at 8 weeks.
Study results demonstrated that considerably more patients who were treated with roflumilast vs vehicle met IGA success at Week 8 (39.9% vs. 6.5%; P<0.001). Various other considerable differences were seen at Week 8 in favor of roflumilast including the reduction in 50% Psoriasis Area and Severity Index as well as Psoriasis Area and Severity Index higher discrimination, 75% reduction, 90% reduction, and 100% reduction. No serious treatment related adverse events were shown, with the most frequent adverse events being diarrhea, headaches, nausea, and urinary tract infection.
Reference: Nagorka C. Roflumilast safe and efficient for patients with chronic plaque psoriasis. Dermatology Times. Updated March 22, 2023. Accessed April 11, 2023. https://www.dermatologytimes.com/view/roflumilast-safe-and-efficient-for-patients-with-chronic-plaque-psoriasis