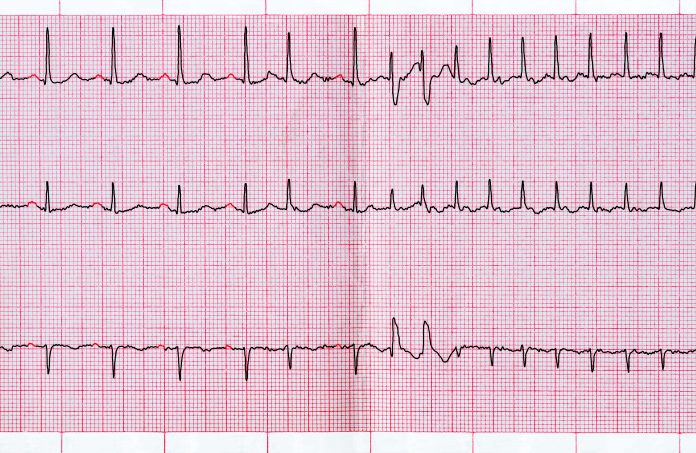
In patients with cardiomyopathy and ventricular tachycardia (VT), early catheter ablation near the time of implantable cardioverter-defibrillator (ICD) placement reduced the combined incidence of recurrent VT, hospitalization, and death compared to ICD alone, reported Dr. Roderick Tung, presenting the results of the PAUSE-SCD trial at the Heart Rhythm Society 2021 meeting (HRS 2021).
Investigators randomized 121 patients with left ventricular ejection fraction (LVEF) <50% and documented sustained monomorphic VT referred for ICD implant to up-front VT ablation versus usual care with antiarrhythmic medications. After a median 31 months of follow up, participants in the ablation arm had 42% lower incidence of the combined primary outcome, driven primarily by fewer recurrent VT events.
Despite recent advances in antiarrhythmic and heart failure therapies, patients with an ICD remain at significant risk for ventricular arrhythmias.1–4 Recurrent VT and associated ICD shocks have been correlated with mortality and worsening heart failure, as well as lower quality of life.2,5,6
Congrats to @DrRoderickTung and team for PAUSE-SCD LBCT showing benefit of first-line VT ablation for reducing recurrent VT
Consistent with results of other trials on preventive approach to VT ablation but now with added inclusion of non-MI VT etiologies. #HRS2021 pic.twitter.com/XwOwG9SrcV
— Jim Cheung (@DrJCheungEP) July 29, 2021
Catheter ablation for VT has been shown to reduce ICD shocks and delay VT recurrence compared to medical therapy.7–9 Prior trials, however, enrolled only patients with ischemic cardiomyopathy, in whom VT arises from well-delineated regions of scar which can be easily identified during ablation procedures.7–9 In non-ischemic cardiomyopathy, with an assortment of underlying conditions and often patchy scar, the efficacy of ablation has been less clear.10,11
How Might PAUSE-SCD Change Clinical Practice?
PAUSE-SCD was the first VT ablation trial to include patients with non-ischemic causes of cardiomyopathy. “It is only the fourth randomized trial to demonstrate improved outcomes from VT ablation compared to control”, added Dr. Tung, “and the first to be performed in Asia with high resolution mapping and epicardial approaches.”
Furthermore, approximately 1/3 of participants had arrhythmogenic right ventricular cardiomyopathy (ARVC). In this challenging disease, ablation data are limited to observational studies, and reported VT recurrence rates are relatively high.12,13
So how might PAUSE-SCD change practice?
“Ablation has historically been viewed as a palliative, last-resort strategy,” stated Dr. Tung in a press release, “and bringing it upfront as concurrent therapy with ICD implantation is a potentially meaningful paradigm shift.”
Importantly, the study enrolled only individuals with monomorphic VT; patients with polymorphic VT or ventricular fibrillation are likely to have different outcomes. Additionally, investigators did not identify a significant reduction in either hospitalizations or mortality alone, and significant reduction in arrhythmia events came at the cost of 8% major procedural complications.
Risk of ventricular arrhythmias varies widely among those with structural heart disease, particularly on a global scale, highlighting the opportunity for future studies to discriminate who will most benefit from early ablation. Analyses from PAUSE-SCD which may be informative include comparison of outcomes in those with higher versus lower LVEF, across the subtypes of cardiomyopathy, and among those receiving amiodarone.8,9
“The SCD-HeFT of VT Ablation Trials”
The PAUSE-SCD investigators also introduced a prospective registry of patients who underwent ablation but declined ICD therapy. This group demonstrated lower VT recurrence rates than those who received ICD with medical therapy, with similar mortality. Though the lack of randomization introduces selection bias (registry participants tended to be younger with higher LVEF), it suggests an opportunity to study ablation-only strategies, particularly in those with non-ischemic cardiomyopathy who may derive less benefit from ICD than those with ischemic cardiomyopathy.14,15
Dr. Tung added that PAUSE SCD can be viewed as “the SCD HeFT of VT ablation trials,” as it included patients with nonischemic cardiomyopathy.
“Nonischemic etiologies are increasingly referred for advanced therapies to mitigate risk for sudden death.” He concluded in a press release: “We hope that these results will influence future guidelines that are trending toward earlier use of catheter ablation over medical therapy in the course of patient treatment.”
References
- Bardy GH, Boineau R, Johnson G, Davidson-Ray LD, Ip JH. Amiodarone or an Implantable Cardioverter–Defibrillator for Congestive Heart Failure. N Engl J Med. 2005;352:225–237.
- Moss AJ, Greenberg H, Case RB, et al.: Long-Term Clinical Course of Patients After Termination of Ventricular Tachyarrhythmia by an Implanted Defibrillator. Circulation. 2004;110:3760–3765.
- Raitt MH, Klein RC, Wyse DG, et al. Comparison of arrhythmia recurrence in patients presenting with ventricular fibrillation versus ventricular tachycardia in the Antiarrhythmics Versus Implantable Defibrillators (AVID) trial. Am J Cardiol. 2003;91:812–816.
- Kloppe A, Proclemer A, Arenal A, et al. Efficacy of Long Detection Interval Implantable Cardioverter-Defibrillator Settings in Secondary Prevention Population: Data From the Avoid Delivering Therapies for Nonsustained Arrhythmias in ICD Patients III (ADVANCE III) Trial. Circulation. 2014;130:308–314.
- Poole JE, Johnson GW, Hellkamp AS, et al. Prognostic Importance of Defibrillator Shocks in Patients with Heart Failure. N Engl J Med. 2008;359:1009–1017.
- Schron EB, Exner DV, Yao Q, et al.: Quality of Life in the Antiarrhythmics Versus Implantable Defibrillators Trial: Impact of Therapy and Influence of Adverse Symptoms and Defibrillator Shocks. Circulation. 2002;105:589–594.
- Reddy VY, Reynolds MR, Neuzil P, et al. Prophylactic Catheter Ablation for the Prevention of Defibrillator Therapy. N Engl J Med. 2007;357:2657–2665.
- Kuck K-H, Schaumann A, Eckardt L, et al. Catheter ablation of stable ventricular tachycardia before defibrillator implantation in patients with coronary heart disease (VTACH): a multicentre randomised controlled trial. The Lancet 2010; 375:10.
- Sapp JL, Wells GA, Parkash R, et al.: Ventricular Tachycardia Ablation versus Escalation of Antiarrhythmic Drugs. N Engl J Med. 2016;375:111–121.
- Dinov B, Fiedler L, Schönbauer R, et al. Outcomes in Catheter Ablation of Ventricular Tachycardia in Dilated Nonischemic Cardiomyopathy Compared With Ischemic Cardiomyopathy: Results From the Prospective Heart Centre of Leipzig VT (HELP-VT) Study. Circulation. 2014;129:728–736.
- Muser D, Santangeli P, Castro SA, et al. Long-Term Outcome After Catheter Ablation of Ventricular Tachycardia in Patients With Nonischemic Dilated Cardiomyopathy. Circ Arrhythm Electrophysiol 9:e004328.
- Daimee UA, Assis FR, Murray B, et al. Clinical outcomes of catheter ablation of ventricular tachycardia in patients with arrhythmogenic right ventricular cardiomyopathy: Insights from the Johns Hopkins ARVC Program. Heart Rhythm United States, 2021; :S1547-5271(21)00407-0.
- Christiansen MK, Haugaa KH, Svensson A, et al. Incidence, Predictors, and Success of Ventricular Tachycardia Catheter Ablation in Arrhythmogenic Right Ventricular Cardiomyopathy (from the Nordic ARVC Registry). Am J Cardiol. 2020;125:803–811.
- Køber L, Thune JJ, Nielsen JC, et al.: Defibrillator Implantation in Patients with Nonischemic Systolic Heart Failure. N Engl J Med. 2016;375:1221–1230.
- 15.Goldenberg I, Gillespie J, Moss AJ, et al. Long-term benefit of primary prevention with an implantable cardioverter-defibrillator. Circulation. 2010;122:1265–1271.
Credit: Original article published here.