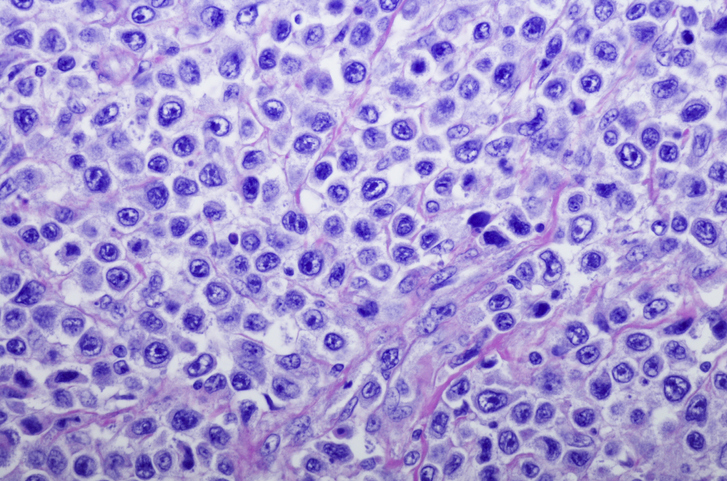
Janssen and Legend Biotech announced new efficacy and safety data for BCMA-directed CAR-T cell therapy cilta-cel in the treatment of relapsed or refractory multiple myeloma. The FDA recently granted priority review of cilta-cel’s biologic license application. The data were presented at this year’s ASCO 2021 Annual Meeting. DocWire News spoke with Saad Usmani, MD, one of the study’s investigators, to discuss these findings.
Credit: Original article published here.